| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 44, pages 995-1009, 1959 NICKEL MINERALS FROM NEAR LINDEN, IOWA COUNTY, WISCONSIN* ALLEN V. HEYL, CHARLES MILTON, AND JOSEPH M. AXELROD U. S. Geological Survey, Washington, D. C. ABSTRACT Nickel minerals occur in comparative abundance as accessories in the Upper Mississippi Valley zinc-lead district of southwestern Wisconsin and northwestern Illinois as well as at other localities in Wisconsin, Illinois, Iowa, Missouri, and Indiana. Millerite is the most common. In southwest Wisconsin millerite is generally associated with violarite and bravoite(?), and near Linden, Wis., all three have been partly altered to a green, yellow, and orange powdery product that is a hydrous basic nickel-iron sulfate, or a series of related sulfates. The alteration product is extremely fine grained and obscurely fibrous; the mean index of refraction is about 1.615 and the birefringence is very low. The x-ray diffraction pattern of the material has broad lines not referable to any known mineral, and chemical analyses indicate that it is essentially a hydrous basic sulfate of nickel and ferric iron. Millerite was deposited near the end of the main period of deposition of the primary sulfides. The other nickel minerals are probably products of oxidation and secondary enrichment of millerite. A hitherto unrecognized type of symmetrical twinning in millerite, suggestive of a lower symmetry than rhombohedral scalenohedral, is described. LOCATION AND GEOLOGIC SETTING Spectroscopic analyses§ of mill products from the Venegar Hill Zinc Company's custom mill at Cuba City, Wis. showed notable quantities of nickel and cobalt in ores of the district. This led members of the field party of the U. S. Geological Survey in cooperation with the Wisconsin Geological Survey to search for nickel and cobalt minerals which they first found and collected in 1946. Laboratory investigations of samples from mines near Linden (Fig. 1) showed primary millerite and secondary violarite, bravoite (?) and a hitherto unrecognized hydrous basic sulfate or a series of sulfates of nickel, iron, and cobalt. Although nickel minerals had not been recognized previously in the ore deposits of the Upper Mississippi Valley, U. S. Grant (1905, written communication) had noted green "copper" stains in the Glanville mine at Linden. The nickel minerals are not common at any locality in the Upper Mississippi Valley zinc-lead district, but are most abundant in the Gilman, Glanville, and Mason mines (Fig. 2). Smaller quantities of millerite and/or violarite have been noted by members of the U. S. Geological Survey at other localities (Fig. 1): (1) James mine, in sec. 9, T.1 N., R. 2 E., near Shullsburg, Wis., (2) Dodgeville No. 2 mine, Dodgeville, Wis., and (3) possibly at the Bautsch mine, 5 miles south of Galena, Ill. Violarite and bravoite (?) as pseudomorphs after millerite were fountd by Allen F. Agnew of the U. S. Geological Survey at the Farrey mine, along Wisconsin State Highway 11, in the NE1/4NW1/4 sec. 14, T. 1 N., R. 1. E., at Leadmine, Wis. (Heyl, Agnew, Lyons, Behre, 1959).
1. Dodgeville No. 2 mine-millerite.
Millerite has a wide distribution elsewhere in the Upper Mississippi Valley, for example: in Lee County, Iowa (Keyes, 1895); in the Chicago, Ill., area (Crook, 1902); from Milwaukee, Wis. (Cleland, 1911, 1). ( Bagrowski, 1940, p. 556-559); in geodes from Iowa and Ill. (Van Tuyl. 1925; Keyes, 1895, p. 401-402); in the St. Louis, Mo., area (Schraut, 1050); in geodes near Columbia, Mo. (Gleason, 1931); and from the Warsaw and Ste. Genevieve limestones of southwestern Indiana (Erd, Evans, Richter, 1957). Bravoite has been found in the Hayden Creek lead mine, southeastern Missouri (Ohle, 1951), and occurs with siegenite, near Fredericktown, Mo. (Rasor, 1943). Millerite with replacement by violarite has recently been identified (1958) by one of the authors, Milton, in Linden County, Mo. associated with galena, sphalerite, chalcopyrite, and barite.
1. Robarts-Hinkel mine.
Primary minerals of the ore deposits are quartz (chert and jasperoid), dolomite, pyrite, marcasite (cobaltian), sphalerite, galena, chalcopyrite, barite, millerite, and calcite. Secondary minerals are smithsonite, limonite, cerussite, gypsum, melanterite, chalcocite, covellite, violarite, and bravoite (?), and the above mentioned sulfate, honessite (Heyl, Milton and Axelrod, 1956). The nickel-bearing zinc-lead ore bodies are groups of banded veins that fill fractures, some of which are faults of small displacement, or are replacements of limestone, shale and dolomite of the Platteville, Decorah, and Galena formations of Middle Ordovician age (Behre, et at., 1950). Near Linden the nickel minerals have been found only in the top member (Quimby's Mill) of the Platteville formation.
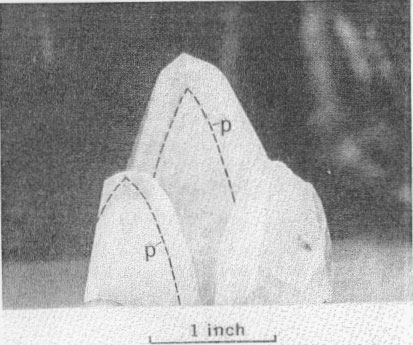
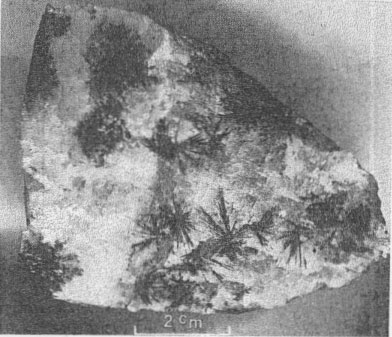
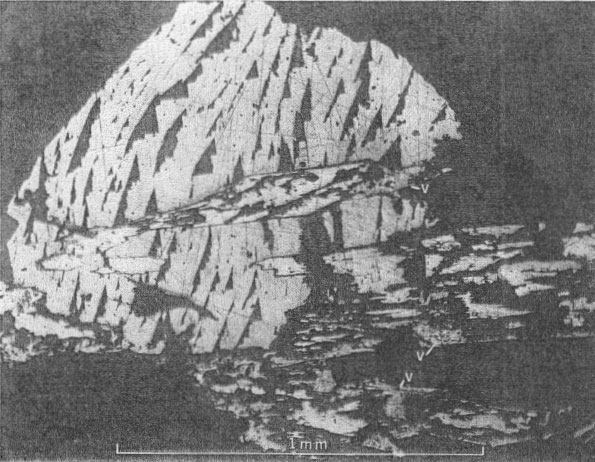
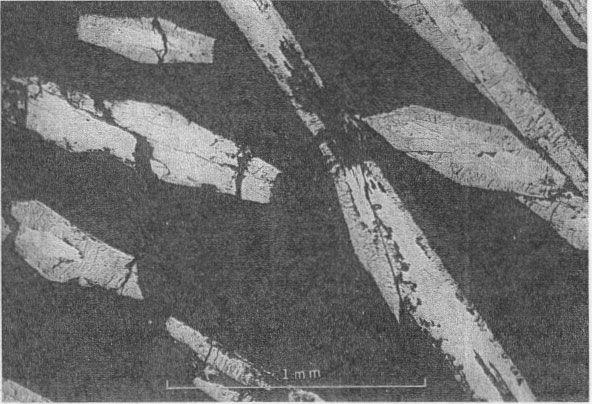
PARAGENESIS OF THE NICKEL MINERALS The sequence of initial deposition of the primary hydrothermal minerals of the Upper Mississippi Valley zinc-lead district is quartz, dolomite, pyrite, marcasite, sphalerite, galena, barite, chalcopyrite, millerite, and calcite. The period of millerite deposition was short, but extended from that of marcasite to that of the first of four habit stages of calcite. The four calcite habit stages can be differentiated as follows: (1) the oldest stage is marked by positive and negative rhombohedrons capped by a basal pinacoid; (2) the next stage shows sharp scalenohedrons, with no basal pinacoid; (3) the third stage is distinguished by stubbier scalenohedrons modified by rhombohedrons; 4) the youngest calcite crystals are complex rhombohedrons with vestigial scalenohedrons. Further, each of the successive habit states shows a decreasing degree of etching, and a progressive decrease in cloudiness of the calcite and in the number of sulfide mineral inclusions. Phantoms showing parts of this succession and oriented progressive overgrowths are common (Fig. 3). Radiating acicular clusters of millerite crystals up to an inch in diameter adhere to earlier deposited sulfides. Other clusters are attached to the walls of small fractures in dolomitized limestone breccias. Milk-white coarsely crystallized calcite of the first or second stage usually surrounds the millerite clusters (Figs. 4 and 5). Octahedral galena, marcasite, sphalerite, and chalcopyrite accompany the millerite and its alteration products. OCCURRENCE OF MILLERITE Millerite, or pseudomorphs of violarite and bravoite (?) after millerite, form radiating acicular clusters of crystals at all the nickel localities in the district. Brassy-yellow millerite is rare; it has been seen only at the Bautsch mine in Illinois, at the Dodgeville No. 2 mine, Dodgeville, Wis. (Fig. 1), and, at the Glanville and Mason mines at Linden, Wis. (Fig 2) where it is partly replaced by violarite. At the other localities the millerite in hand specimens is dark gray-black, though it is unmistakable in polished section. When not completely oxidized, it is usually coated with dark-gray violarite, which is commonly associated with bravoite (?). The relations of these nickel minerals are shown in Fig. 4, 5, 6, 7, 8, and 9.
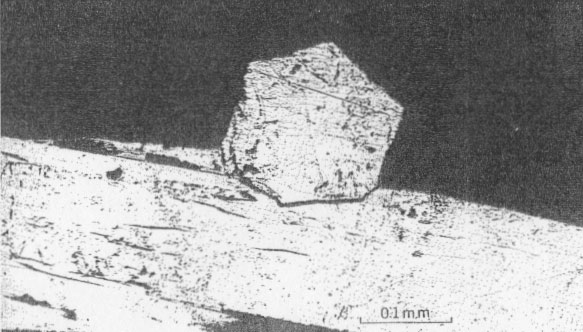
Millerite occurs as acicular crystal inclusions in bands in calcite, commonly in late calcite of stage one or in early calcite of stage two. The relations of millerite to octahedral galena crystals are shown in Figs. 6 and 7. The youngest galena commonly is in octahedrons, which may be perched upon millerite crystals or enclose them. Rarely is millerite surrounded by a hull of chalcopyrite, as shown in Fig. 12. Chalcopyrite more commonly occurs as bands of small discrete tetrahedrons closely associated with millerite within the first or second stage calcite. The millerite shows a remarkable symmetrical twinning, not previously noted in the literature (Figs. 8, 9, and especially 10, 11), which suggests a possible lower symmetry than rhombohedral scalenohedral class of the hexagonal system. Buerger (1947) has indicated a similar possibility in the case of dihexagonal dipyramidal pyrrhotite.
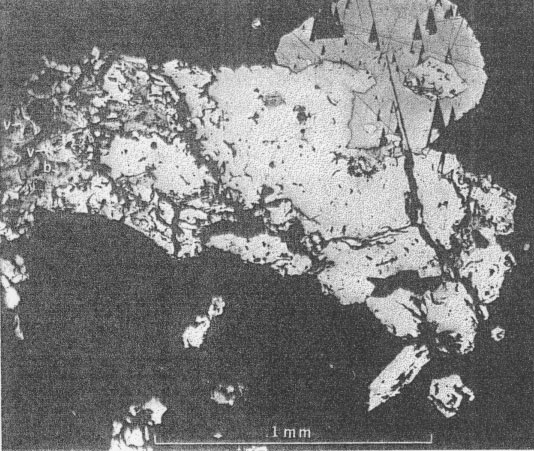
OCCURRENCE OF VIOLARITE AND BRAVOITE (?) Much of the millerite has been replaced by violarite and bravoite (?). These minerals are most common near Linden, Wis. (Fig. 2), but have also been found in some abundance at the Farrey mine at Leadmine, Wis. (Fig. 1), and uncommonly at the James mine near Shullsburg, Wis. They occur as dark-gray partial or complete replacements of millerite (Fig. 6), as coatings or overgrowths on millerite (Figs. 6 and 7), as microcrystal molds or clusters surrounding an acicular cavity that formerly held millerite (Fig. 7), and as acicular pseudomorphs after millerite crystals. Violarite and bravoite (?) commonly replace or coat only those parts of the millerite crystal not enclosed by other sulfides such as galena (Figs. 6 and 7). Violarite is identified by a diagnostic x-ray pattern, and in polished sections by a light violet-gray color, hardness (4˝-5˝) and the presence of marked cleavage {001} . It is distinguished from millerite by its color, lower reflectivity, isotropism, and greater porosity (Figs. 8 and 9). Bravoite (?) occurs sparingly in discrete grains with sharp planar boundaries (Fig. 6). It is always associated with and commonly surrounded by violarite. The bravoite (?) is distinguished from violarite in polished sections by its darker violet-gray color, its greater hardness, and lack of cleavage. It is in too small a quantity to identify with certainty.
Violarite and the associated bravoite (?) are unquestionably younger than millerite for they appear always to replace or coat acicular millerite crystals (Figs. 5, 6, 7, 8, 9, and 10). At all known occurrences of millerite near Linden (2) the nickel-iron sulfides are found in partly oxidized zinc-lead deposits, presumably in the zone of former fluctuations of the water table, suggesting that both minerals may be supergene sulfides formed by the oxidation of millerite, but not precluding their hydrothermal origin.
Ramdohr (1955), discussing the relations of millerite and linnaeite (in which group he includes violarite), observes that linnaeite-violarite commonly is replaced by millerite as an exsolution product; whereas the opposite, replacement of millerite by linnaeite-violarite, is rare. The relations described by us cannot be interpreted otherwise than as replacement of millerite by violarite.
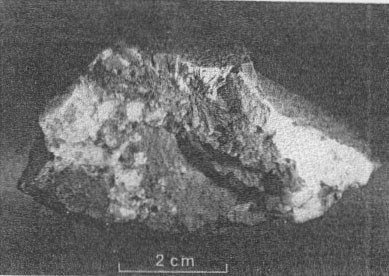
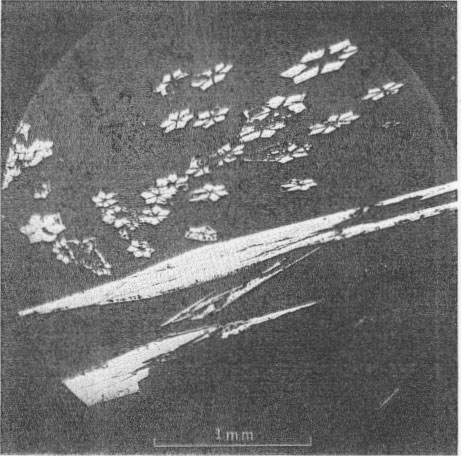
TEMPERATURE OF DEPOSITION OF THE ORES The temperature of deposition of the primary ore minerals of the Upper Mississippi Valley lead-zinc district has been investigated by several methods; Newhouse (1933, p. 744-750), studying liquid inclusions in sphalerite, found the temperature of deposition to be from 80° to 105° C.; Garrels (1941), considering the zoning relations of sphalerite and galena, assumed concentrated chloride solutions at 100° C. under 60 atmospheres pressure; Bailey and Cameron (1951), examining liquid inclusions in calcite and sphalerite, found temperatures of formation from 75° to 121° C. Millerite presumably would be deposited at temperatures near the lower end of this range, as it is one of the last minerals deposited. Recent work by Kullerud (1953), however, based on the Zn-Fe ratio of sphalerite from the district indicates minimum temperatures of deposition for the southwestern Wisconsin deposits from less than 138° to 245° C. Field relations (Behre, Heyl, and McKnight, 1950, p. 51-69) support the lower temperatures suggested by Newhouse, Garrels, and Bailey and Cameron, rather than the higher temperatures proposed by Kullerud. OXIDATION OF NICKEL SULFIDES Millerite, violarite, and bravoite(?) have been altered, presumably by weathering, at many of the localities to an orange-red, yellow, or green nickel sulfate (Fig. 4) (or sulfate series). The sulfate, found only in the zone of oxidation, at (or near) the fluctuating ground water table, replaces the nickel sulfides and also forms thin crusts along cleavage planes of calcite near the original millerite clusters (Fig. 4). This sulfate is insoluble in water but is soluble with slight effervescence in dilute acid solutions; free oxygen is liberated. The movement of nickel during redeposition as secondary sulfides rarely exceeds 1 mm., but crusts of the secondary sulfate mineral extend from 1 to 8 mm. beyond the original millerite crystals. Thus, nickel seems to have been more soluble in the probably acid solutions that deposited the sulfate mineral than in solutions that deposited violarite and bravoite (?).
Millerite has been found near Milwaukee, Wis. (Bagrowski, 1940, p. 556-559) and Keokuk, Iowa (Keyes, 1895, p. 401-402). Both of these localities are near but beyond the limits of the lead-zinc district. At Milwaukee, a green oxidation product of millerite called "zaratite," reported by Bagrowski (1940, p. 556-559), may be similar to one of the variations of the oxidation product at Linden. The occurrence of a green to yellow oxidation product of millerite in Indiana has been noted by Richard C. Erd of the U. S. Geological Survey. X-ray patterns, however, are different from that of the sulfate from Linden, Wis. (Richard C. Erd, 1953, oral communication). Howarth (1930, p. 3-5) mentions two oxidation products of millerite from a coal mine in Monmouthshire, Wales. One of these is a yellowish-green soluble nickel sulfate, probably morenosite; the second is a "sulfate insoluble in water but soluble in dilute mineral acid, presumably a basic nickel sulfate although there was insufficient material for qualitative analysis." Possibly this second oxidation product is the same as that which we call honessite. PHYSICAL AND CHEMICAL PROPERTIES OF SECONDARY NICKEL SULFATES The variation in color of the secondary nickel sulfate from orange to red, to yellow to green is thought to correspond to varying degrees of oxidation of the iron present. The varieties that are orange-red and yellow may be richer in ferric iron than the variety that is green. The material may actually be a series connected by FeO-Fe2O3 variation as in the frondelite-rockbridgeite series, or by variations in hydrations. Microscopic examination of the pseuomorphs after millerite shows the material to be extremely fine grained and not uniform. There is an obscurely fibrous structure, with positive elongation and extinction at about 12°. Even under high magnification very few areas are free of turbidity, and these are too small for conoscopic study. The mean index of refraction is about 1.615, and the birefringence is very low. No visible differences are apparent between the varicolored particles, except color. In an attempt to clarify the crystalline structure of the unknown nickel sulfate, Edward Dwornik of the U. S. Geological Survey kindly prepared electron micrographs. Some of the grains suggest a stacked or layered structure similar to kaolin or mica, but the lack of indication of crystal form in the rest of the grains suggests that it is a substance of low crystallinity. X-ray diffraction patterns from numerous samples of the material prepared by Axelrod had broad lines not referable to any known material. Besides sharp lines referable to known materials present, such as calcite, cerussite, and millerite, there were:
The x-ray diffraction pattern of "anodic nickel hydroxide," reported by Klug and Alexander (1954, Fig. 6-27c) is very similar to that of our material. If this material consists partly of trivalent nickel, honessite may be isomorphous with it, with trivalent iron replacing trivalent nickel. K. J. Murata of the U. S. Geological Survey made a spectrographic analysis of the green material and found Ni, Ca, Fe, Co, and minor Pb, Mg, Mn, Si, and Zn (?). Microchemical analyses were subsequently made on three different samples of 15 to 25 mg. in weight isolated from the same source material from Linden, Wis.
* Total iron calculated as Fe2O3.
The material apparently is essentially a hydrous basic sulfate of nickel and ferric iron, and CaO is a major or minor constituent. The three analyses are by no means conclusive as to the composition of the material. Considering the nature of the material, and the small quantities analyzed (15-25 mg.), however, there is more or less agreement. Cobalt in the secondary nickel sulfate may be derived in part from the oxidation of cobalt- and arsenic-bearing marcasite accompanying the millerite. Complex intergrowths of marcasite and a possible cobalt-arsenic mineral of somewhat similar optical properties were noted in 1946 by Milton during metallographic examinations of marcasite from the Martin mine, Benton, Wis. Spectroscopic examination suggested a mineral of the CoAsS (cobaltite), or CoAs2 (safflorite) type. Arsenic-bearing ores have been noted from the Linden area (Charles Singer, 1947, oral communication), where the arsenic probably occurs as this cobalt-arsenic mineral with marcasite. The chemical literature, summarized in Mellor (1936, p. 462) lists many syntheses of basic nickel sulfates. It is not possible, however, to identify either these sulfates or the Wisconsin substance precisely enough to say that any one of them has a particular formula. Attempts to synthesize a compound having properties like those of the Wisconsin mineral were unsuccessful. The oxidation product, thought to be a hydrated nickel-iron sulfate, and tentatively named honessite (Heyl, Milton, Axelrod, 1956) after the late Professor Arthur P. Honess of Pennsylvania State University, will require further work on better material than that available to us to define it precisely. We hope that the present admittedly unsatisfactory description will serve to bring this substance to the attention of other workers who may clarify its status as a new mineral. ACKNOWLEDGMENTS Although the paper is a joint effort, the first author, Heyl, is mainly responsible for the collecting of samples and descriptions of the geologic setting, paragenesis and field relations of the minerals. Milton did most of the detailed mineralogic studies and interpretations, and Axelrod is responsible for the x-ray studies. The authors wish to acknowledge the assistance of many members of the U. S. Geological Survey in the preparation and review of the manuscript, especially R. C. Erd who also spent many hours in discussion and research on the mineralogy of these minerals. A. F. Agnew, A. E. Flint, C. E. Brown and J. W. Allingham of the U. S. Geological Survey kindly collected additional samples of the minerals for study. REFERENCES BAGROWSKI, B. P. (1940), Occurrence of millerite at Milwaukee, Wisconsin: Am. Mineral., 25, 556-559. BAILEY, S. W. AND CAMERON. E. N. (1951), Temperatures of mineral formation in bottomrun lead-zinc deposits of the Upper Mississippi Valley, as indicated by liquid inclusions: Econ. Geol., 46, 626-651. BEHRE, C. H., JR., HEYL, A. V., JR., AND MCKNIGHT, E. T. (1950), Zinc and lead deposits of the Mississippi Valley, in Dunham, D. C., ed., Part 7, Symposium and proceedings of Section F., the geology, paragenesis, and reserves of the ores of lead and zinc, 51--69. BUERGER, M. J. (1947), The cell and symmetry of pyrrhotite: Am. Mineral., 32, 411-414. CLELAND, H. F. (1911), The fossils and stratigraphy of the Middle Devonian of Wisconsin: Wisconsin Geol. and Natl. History Survey, Bull., 21, 6. CROOK, A. R. (1902), The mineralogy of the Chicago area: Chicago Acad. Sci., Natl. History Survey Bull., 5, 55. ERD, R. C., EVANS, H. T., JR., AND RICHTER, D. H. (1957), Smythite, a new iron sulfide, and associated pyrrhotite from Indiana: Am. Mineral., 42, 309-333. GARRELS, R. M. (1941), The Mississippi Valley type lead-zinc deposits and the problem of mineral zoning: Econ. Geol., 36, 729-744. GLEASON, C. D. (1931), Rare minerals in Missouri: Compass, 11, 132-134. HEYL, A. V., JR., AGNEW, A. F., LYONS, E. J., AND BEHRE, C. H., JR. (1959). The geology of the zinc and lead deposits of the Upper Mississippi Valley: U. S. Geol. Survey Prof. Paper 309, in press. HEYL, A. V., MILTON, CHARLES, AND AXELROD, J. M. (1956), Nickel minerals from near Linden, Wisconsin: Geol. Soc. America Bull. (abs.), 67, 1706. HOWARTH, W. E. (1930), Millerite: Rocks and Minerals, v. 5, no. 1, pp. 3-5. KEYES, C. R. (1895), Geology of Lee County, Iowa: Iowa Geol. Survey Second annual -- report, 3, 401-402. KLUG AND ALEXANDER (1954), X-ray diffraction procedures: John Wiley and Sons, New York, Fig. 6-27C, p. 385. KULLERUD, GUNNAR (1953), The FeS-ZnS system as a geological thermometer: Norsk Tidskrift, 32, 61-147. MELLOR, J. W. (1936), Modern inorganic chemistry, XV: London, Longmans, Green, p. 462. NEWHOUSE, W. H. (1933), The temperature of formation of the Mississippi Valley lead-zinc deposits: Econ. Geol., 28, 744-750. OHLE, E. L. (1951), The geology of the Hayden Creek lead mine, southeastern Missouri labs]: Econ. Geol., 46, 111-112. RAMDOHR, PAUL (1955), Die Erzmineralien and ihre Verwachsungen: Berlin, AkadVerlag., p. 480. RASOR, C. A. (1943), Bravoite from a new locality: Econ. Geol., 38, 399-407. SCHRAUT, J. A., JR. (1950), The occurrence and association of millerite and fluorite in limestone quarries of the St. Louis, Missouri, area: Rocks and Minerals, 25, 134-135. VAN TUYL, F. M. (1925), The stratigraphy of the Mississippian formations of Iowa: Iowa Geol. Survey, 30, 304-346. NOTES * Publication authorized by the Director, U. S. Geological Survey. § Spectroscopic analyses by J. C. Rabbit, U. S. Geological Survey, March, 1943.
Manuscript received December 10, 1958.
|