| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | Contacts |
|
|
|||||||

|
|
Volume 27, pages 614-628, 1942 RUSSELL G. WAYLAND, U. S. Geological Survey, Washington, D. C. ABSTRACT New data on rhodochrosite are presented, including unpublished analyses. Seventy-nine analyses are calculated to MnCO3, FeCO3, MgCO3 and CaCO3 and are plotted in three ternary diagrams. The frequency of appearance of Ca, Fe, and Mg in significant quantities as isomorphous constituents of the 79 rhodochrosite specimens is found to be in the ratio 21:17:12. Complete isomorphous miscibility with calcite and siderite is demonstrated. The series to magnesite is incomplete. The refractive indices and specific gravities of rhodochrosite are shown to vary directly with the composition, and to be calculable from the proportions of the four end members of the system. The composition of a specimen, however, cannot be deduced from the specific gravity and optical properties alone. The nO of pure MnCO3 is shown to be 1.816, and the specific gravity is 3.70. The theoretical specific gravity calculated from the molecular weight and the volume of a unit cell is considerably larger. Rhodochrosite from Butte, Montana, is described and analyses of rhodochrosite and ankerite from Butte are given. INTRODUCTION In 1917 W. E. Ford1showed that the refractive indices and specific gravities of members of the calcite group of minerals could be calculated directly from their percentage composition with reasonable accuracy. Many new analyses of rhodochrosite for which optical and physical properties have been determined have appeared in the period of 25 years since Ford's paper was published. The interest in rhodochrosite as a source of manganese has been heightened by the present emergency, and it was in the course of an investigation of manganese reserves at Butte, Montana, with Charles F. Park, Jr., that the writer decided that further quantitative study of some of the properties of rhodochrosite might be worthwhile. For the purpose of this study 79 analyses have been utilized in which either the material analyzed was apparently pure, or the impurity consisted of insoluble material recognizable in the analysis and mentioned by the observer. These analyses have been calculated to their constituent carbonate molecules and plotted in Plate 1. With a few exceptions, they contain MnCO3 in excess of all other carbonate molecules. Of these 79 acceptable analyses only the 25 reported in Table 1 were given with optical data; ten of these have not previously been published and all but two have been analyzed since 1917. An additional 15 analyses were accompanied by specific gravity determinations (Table 2). Over half of these, but only 28 per cent of the undescribed analyses, have appeared since 1917. Hence the trend toward correlation of chemical and physical data in mineralogical studies is noticeable in the rhodochrosite series, just as it is in other series which have been receiving attention in recent years. Nevertheless, the relative scarcity of high grade analyses of pure, clean material, accompanied by carefully determined physical data, is distinctly evident in the rhodochrosite series. Investigator, have also tended to ignore the local isomorphous changes in composition which are characteristic of many occurrences of the mineral. Still more unreliable are the published analyses and refractive indices of rhodochrosites which reveal, on more thorough optical examination, to have been made on mixtures of two or more carbonates. The writer has omitted a few "rhodochrosites" from consideration because of this fact. COMPOSITION The principal carbonate molecules present in rhodochrosite are MnCO3, CaCO3, FeCO3, and MgCO3. In a few specimens ZnCO3 is a constituent, and one analysis contains cadmium in an unidentified form. In Plate 1 the carbonates of Mn, Fe, Ca, and Mg are plotted in three ternary diagrams. A mineral appears in two diagrams if its third and fourth most abundant metals are about equal in quantity, and it appears in all three fields if Ca, Mg, and Fe are equally abundant. The three diagrams of Plate 1 can be considered as portions of the three faces of a tetrahedron that join in an apex considered to represent 100 per cent MnCO3 Of the 79 analyses plotted in Plate 1, 56 are either three-component carbonates or they contain a fourth metal in such small quantity that it may be neglected. In plotting, this fourth metal is added to the others according to the following scheme: if Ca, it is added to Mg; if Fe, it is added to Mn; and if Mg, it is distributed ¾ to Ca and ¼ to Mn. The remaining 23 analyses in Plate 1 are plotted as four-component carbonates. Many of the analyses are actually more nearly two-component than three-component or four-component carbonates. These lie near the edges of the tetrahedron whereas the three and four-component analyses lie, respectively, on the triangular faces or well within the trihedral angle forming the apex of the tetrahedron. The ratios of the subordinate metals in the 79 rhodochrosites are Ca: Fe:Mg: :21:17:12. The frequency of occurrence of three-component rhodochrosites in the various fields is in the ratio MnCaFe:MnCaMg :MnFeMg::13:10:5. These ratios should not be considered of great significance because the analyses do not constitute a representative sample of all known rhodochrosites, nor are they proportioned correctly among the districts that they represent. ISOMORPHISM The ionic radii of bivalent Mn, Fe, Ca, and Mg as given by various investigators are listed below. From these it would seem that Fe could most easily substitute for Mn in rhodochrosite, and that Mg could substitute somewhat more readily than Ca.
a Goldschmidt, V. M., Faraday Soc. Trans., 25, 253 (1929). The MnCO3-FeCO3 series appears to be completely miscible. Ford2 had noted a considerable break in the series between 50 and 70 per cent MnCO3, but analyses H and J from Ouray and Leadville, Colorado, fall in this zone. In the MnCO3-CaCO3 series Ford3 deduced from a study of molecular volumes, rhombohedral angles, and analyses, that only comparatively small amounts of other molecules would be found replacing CaCO3. Of these he considered MnCO3 the most likely to replace CaCO3 isomorphously because of the closest relation between molecular volumes and plane angles of the rhombic faces. By means of an x-ray study and new analyses, Krieger4 later showed complete isomorphism in the series from CaCO3 to analysis Z which is 42 per cent MnCO3. The series is completed through specimens T from Batesville, Y from Japan, X from Tennessee and others here assembled, indicating that Ca and Mn may substitute for each other in any proportion. Specimens T and X were studied optically by the writer and were seen to consist essentially of single carbonate minerals, variable within limits but not composite. However, it is well to point out that many of these analyses which complete Krieger's series are not of the very best material. Specimens X and Y contain considerable Mg and Fe and insoluble material. Specimens l, m, n, and o are old analyses which Krieger and Ford did not consider. Ford mentions specimen l but classes it as exceptional. In summary, the MnCO3-CaCO3 series is well demonstrated at the manganocalcite end by Krieger, and somewhat substantiated in the range from 42 to 85 per cent MnCO3 by the present study. The series MnCO3-MgCO3 is incomplete. The magnesite highest in manganese quoted by Ford5 contains 13.33 per cent of MnCO3 and 16.99 per cent FeCO3. Twelve other analyses of manganoan magnesite contain less than 3.5 per cent MnCO3. At the rhodochrosite end of the MnCO3-MgCO3 series, with the exception of specimen p, the highest MgCO3 content is 9.4 per cent in specimen V. In only one rhodochrosite in nine is MgCO3 the second most abundant constituent. Specimen p (Table 2) with 27.5 per cent MgCO3 is from Hambach (Nassau), Germany, and was analyzed in connection with a study of the thermal dissociation of rhodochrosite. The material is not described, but the analysis should probably be accepted because the oxides calculate directly to carbonates and the only impurity seems to be 0.74 per cent SiO2. REFRACTIVE INDICES The observations of the writer indicate that many natural rhodochrosites vary in their composition and optical properties more than seems hitherto to have been recognized. This is especially true of rhodochrosite from Butte, Montana, but is equally true of many specimens from elsewhere that have come to the writer's attention. Most banded rhodochrosites have small changes in composition from band to band. Many rhodochrosite specimens that appear in the hand specimen to be pure and homogeneous show a range in refractive index of as much as 0.010 in the space of an inch or so. Single crystals may be zoned. Commonly it is not enough to make only one observation of refractive index on a single grain of an analyzed sample. Some published descriptions give indices to 0.0001 but are actually questionable at 0.01. In Table 1 those observations which were found by the writer to have been made on variable material are reported as a range rather than a single observation or an average.
The refractive indices assumed for all end members in the three ternary systems studied in this paper are:
Fig. 1. Observed and calculated refractive indices. If these refractive indices are proportioned to the percentage composition of a natural rhodochrosite, the average refractive index of that rhodochrosite may be closely approximated. That this is true is shown by Table 1 and by Fig. 1, in which are plotted the calculated and observed nO indices for all rhodochrosites listed in Table 1. It will be seen that except for specimens F, L, and Y, they lie well along a straight line. Specimens L and Y are described as dense and turbid, hence they were likely difficult to study optically. Specimen F is unaccountably low. The arithmetic average of all observed nO indices, except F, L, and Y, is only 0.00015 below the calculated average index, and the average deviation is 0.00125. Ford,6 on the basis of the limited optical data available in 1917, gives a value of 1.817 for the nO of pure MnCO3. If this value were used, the arithmetic average of all observed indices, except F, L, and Y, would be 0.00080 below the calculated index and the average deviation 0.00140. Hence the value 1.816 for pure MnCO3 is more nearly correct than 1.817.
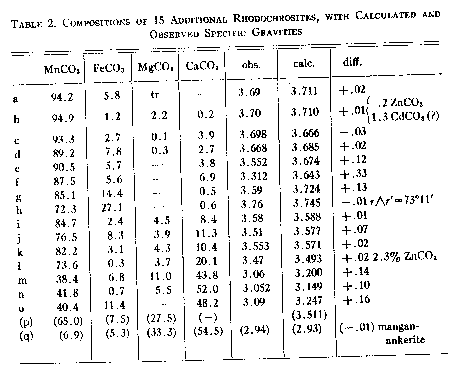
A. Larsen and Wherry, Jour. Wash. Acad. Sci., 7, 365-368 (1917); from the John Reed mine, Alicante, Lake County, Colorado, transparent crystals, r^r'=73°10'-20', anal. Wherry. B. Sundius, Geol. Fören. Förh., 47, H. 1, pp. 269-270 (1925); from Alma; Colorado, r^r'=73°2', anal. Bygden. Hedvall, ibid., pp. 73-80, finds thermal decomposition begins around 200° C. and dissociation is complete in the range below 540° C. C. Gaubert, Bull. Soc. Franc. Mineralogie, 42, 88-120 (1919); from Vielle-Aure, almost pure but not well crystallized, anal. Gruner. D. Ortloff, Zeits. phys. Chem., 19, 215 (1896); from Biersdorf. E. Baric and Tocan, Annales Geol. de la Peninsule Balkanique, Belgrad, 8, 129-130 (1925); from Ljubija, Bosnia, rosecolored secondary rhodochrosite on limonite. F. Harada (Japan. Jour. Assoc. Min. Pet. Econ. Geol., 1932), Neues Jahrb. Min., Geol., Ref. 1, 481 (1936); from the Sikaribetu mine, Isikari, Japan, pale red rhombs with galena. G. Ford, Trans. Connecticut Acad. Arts and Sci., 22, 211-248 (1917); from Branchville, Conn., anal. Bradley. H. Burbank, U. S. Geol. Survey Bull. in preparation; from the Bachelor mine, Ouray, Colorado, salmon-pink crystalline, zoned rhodochrosite in quartz veins with sphalerite and pyrite, 2.38 per cent insoluble, anal. Murata, optical data, Wayland. J. Mayo and O'Leary, Am. Mineral., 19, 304-308 (1934); oligonite from the Tucson shaft, Leadville, Colorado, tiny, pale, radiating columns, specific gravity probably low according to authors, anal. O'Leary. K. Callaghan, Econ. Geology, 33, 508-521 (1938); from the Black Boy mine, Drum Mountains, Utah, banded pink rhodochrosite, cementing dolomite breccia, anal. Bailey, optical data, Glass. Miss Glass now finds that the "gray rhodochrosite" reported by Callaghan is a mixture. L. Yosimura, Jour. Fac. Sci., Hokkaido Imp. Univ., series 4, 4, 313-451 (1939); from the Kaso mine, Japan, dense turbid rhodochrosite with SiO2, A12O3, H2O, and insoluble totalling 10.95 per cent. M. Yosimura, op. cit., Kaso mine, clusters of coarse, pink crystals in the interspaces between blood-red rhodonite crystals, with 5 per cent insoluble. N. Emma mine, Butte, Montana, deep pink, coarse, zoned cleavage rhomb, anal. Fairchild, optical data, Wayland. O. Ross, C. P., U. S. Geol. Survey Bull. in preparation; from Whitlatch dump, Austin, Nevada, 15.08 per cent insoluble, 1.04 per cent Fe2O3, anal. Steiger, optical data, Glass. P. Ross, op. cit., from Belle Wilder, Austin, Nevada, 24.45 per cent insoluble, anal. Steiger, optical data, Glass. Q. Ross, op. cit., from Cummings lease, Austin, Nevada, 32.03 per cent insoluble, anal. Steiger, optical data, Glass. R. Ross, op. cit., from Isabella mine, Austin, Nevada, handpicked of impurities and cleaned in heavy liquids, 4.58 per cent insoluble, anal. Wells, optical data, Glass. S. Ross, op, cit., from Ruby Incline, Austin, Nevada, 26.12 per cent insoluble, anal. Steiger, optical data, Glass. T. Miser and Hewett (U. S. Geol. Survey, Bull. 921-A, in preparation), U. S. Geol. Survey, Bull. 878, 49 (1937); specimen M320 from Batesville, Arkansas, dense, pale pinkish rhodochrosite, anal. Wells, optical data, Wayland. Indices of most grains of the sample lie between 1.79 and 1.80. Other analyses reported are composite. U. Goddard, U. S. Geol. Survey unpublished analysis, 1940; from Algonquin mine, Philipsburg, Montana, anal. Fahey, optical data, Wayland. V. Goddard, op. cit., from Algonquin mine, anal. Fahey, optical data, Wayland. W. Goddard, op. cit., from Scratch Awl mine, Philipsburg, Montana, anal. Fahey, optical data, Wayland. X. Keith, in U. S. Geol. Survey, Bull. 878, 95 (1937); from Sevier, Tennessee, impure, zoned, turbid rhodochrosite, 15.54 per cent SiO2, 2.42 Al2O3 anal. Steiger, optical data, Wayland. Y. Yosimura, op. cit., from the Kaso mine, Japan, dense grayish rhodochrosite replacing rhodonite; with sericite and quartz. Turbid, pleochroic, impure, with SiO2, A12O3, H2O and insoluble totalling 19.26 per cent. Z. Krieger, Am. Mineral., 15, 23-29 (1930); from Sparta, North Carolina, anal. Shannon. The nE indices calculated from Ford's value of 1.597 for pure MnCO3 differ an arithmetic average of 0.0064 from observed indices, if specimen F is disregarded. The average deviation is 0.0032. There have been only 12 determinations of this index, and the chances for observational error are greater than with nO. SPECIFIC GRAVITY Of the 26 analyzed specimens of rhodochrosite (Tables 1 and 2) for which specific gravities have been observed, 17 have been published since 1917 or make their appearance in this paper. Previous to Ford's paper7 the specific gravity of rhodochrosite was generally given at 3.45 to 3.60. As Fig. 2 shows, some natural rhodochrosites do lie in this range. The value of 3.70 assumed by Ford for the specific gravity of pure MnCO3 is substantiated by the recent additional observational data and by calculation from specific refractive indices of MnO and CO2 and the observed index of MnCO3 but it is considerably lower than the theoretical specific gravity derived from x-ray data, as will be shown. The specific gravities assumed for the end members in the three ternary systems are Ford's: FeCO3 3.89 MnCO3 3.70 MgCO3 2.96 CaCO3 2.715 In calculating specific gravities for specimens b and l the specific gravities of ZnCO3 and CdCO3(?) were taken as 4.45 and 5.6, respectively. Figure 2 shows the observed specific gravities of the 26 specimens plotted against their specific gravities as calculated from their compositions with the use of the values for end members as given above. In a number of specimens the observed specific gravity falls short of the calculated specific gravity, and in many others they are equal, but in none are they substantially greater. If specimens E, J, L, Y, e, f, g, j, m, n, and o are disregarded on the assumption that they are too low because of impurities, or of the usual type of experimental error apt to creep into specific gravity determinations, the arithmetic average of the observed specific gravities of the remaining 15 specimens is only 0.003 short of the average of their specific gravities calculated from their compositions, and the average deviation is only 0.015.
a. Kunz, Am. Jour. Sci., 3d series., 34, 477-478 (1887); from the John Reed mine, Alicante, Lake County, Colorado, rich red, transparent rhombohedrons, pleochroic, nO salmon pink, nE light yellow, anal. Mackintosh. b. Zsviny (Földtani Közlöny, Budapest, 1927), Min. Abs., 3, 508 (1928); from Rákos, Slovakia, small scalenohedrons on limonite. c. Baric and Tucan, Annales Geol. de la Peninsule Balkanique, Belgrad, 8, 129-130 (1925); from Ljubija, Bosnia, light rose-colored secondary rhodochrosite on limonite. d. Barn and Tutan, op. cit., from Ljubija, Bosnia, brown rhodochrosite on limonite. e. Vesely (Rozpravy, Ceské Akad., 1922), Min. Abs., 2, 142 (1923); from Litosice, Bohemia. f. Vesely, op. cit., from Chvaletice, Bohemia. g. Sandberger, Neues Jahrb. Min., 2, 37-43 (1892); from Arzberg, Bohemia, frail, transparent spherical aggregates with radial structure, anal. Hilger. h. Brush and Dana, Am. Jour. Sci., 3d series, 18, 50 (1879); from Branchville, Conn., pink rhodochrosite in lithiophilite, and white or slightly pink granular masses with apatite and quartz, anal. Penfield. i. Yosimura, Jour. Fac. Sci., Hokkaido Imp. Univ., series 4, 4, 313-451 (1939); from the Kaso mine, Japan, pink rhodochrosite ore with tephroite, 2.7 per cent SiO2. j. Yosimura, op. cit., from the Kaso mine, Japan, pink rhombohedra in drusy quartz veins, 2 per cent insoluble. k. Kersten, (Jour. pr. Chem., 37, 163, 1887), Dana System, V ed., 691(1884); from Voightsberg, Saxony. 1. Browning, Am. Jour. Sci., 3d series, 40, 375-376 (1890); from Franklin Furnace, N. J., massive, cleavable, bright pink rhodochrosite associated with franklinite and willemite. m. Bukovsky, Zeits. Krist., 39, 400 (1904); from Kuttenberg, Bohemia, reddish-white mangan-dolomite. n. Roepper, Am. Jour. Sci., 2nd series, 50, 37 (1870); from Stirling Hill, Franklin Furnace, N. J., delicate pink cleavage masses, with willemite. o. Weibull, Tsch. Min. Petr. Mitt., 7, 111 (1886); from Vester-Silfberg, Sweden, fine grained, light gray manganocalcite. p. Manchot and Lorenz, Zeits. anorg. Chem., 134, 297-316 (1924). q. Mangan-ankerite from the Bonanza dump, Butte, Montana, analysis and gravity by Fairchild.
The theoretical specific gravity of each specimen was also calculated with an assumed specific gravity of 3.71 for pure MnCO3 The fifteen best determinations were then found to average 0.013 below their calculated specific gravities. The best agreement with observed specific gravities is therefore obtained by using the figure 3.70 for pure MnCO3 The theoretical specific gravity of pure MnCO3, may be calculated by two independent methods. Application of the law of Gladstone and Dale, G = n-1/K, where n is the mean refractive index of MnCO3 {cubed root of (ω2ε)} and K equals the sum of the specific refractive indices of MnO and CO2 times their respective weight percentages, gives a value of 3.707 for pure MnCO3,
(Mol. wt.) (No. of mols./unit cell) Brentano and Adamson8 measured a rhodochrosite on which they had observed the specific gravity to range between 3.47 and 3.76, indicating that the material ranged in compostion from ferroan to calcian or magnesian rhodochrosite. The length of an edge, ao', of a unit cell corresponding to a cleavage rhomb was found to be 6.064Å, and the angle α' of a rhombic face was 102°50'. Such a unit cell contains four molecules of MnCO3. From this data Brentano and Adamson calculated a specific gravity of 3.747. They may have measured a ferroan portion of their sample. The true unit cell of rhodochrosite and of other members of the calcite group has been found by Wyckoff9 not to correspond with the cleavage rhombohedron but to be a steep rhombohedron which contains only two molecules. Wyckoff obtained some of the analyzed material of specimen A from Lake County, Colorado, and determined the length of the edge of its unit rhombohedron, ao, to be 5.61Å and the rhombic face angle a to be 47°46'. Later Wyckoff changed the ao value to 5.84Å.10 Using the values 5.84Å and 47°46' for the Lake County rhodochrosite and calculating the molecular weight from the analysis, the writer obtained a density of 3.785. This is higher by 0.075 than the observed specific gravity, 3.710. Bragg11 and Wyckoff12 in their latest compilations on crystal structure give the following dimensions for MnCO3:
ao
α
ao'
α' Using the values 5.84Å and 47°20' the writer obtained a theoretical specific gravity of 3.851, and using the values 6.01Å and 102°50' the specific gravity is 3.837. These are much higher than observed values for rhodochrosite. If we now assume that the specific gravity of pure rhodochrosite is 3.70 and its nO is 1.816, and that the physical properties of natural rhodochrosites can be calculated from the chemical compositions, we can place on Plate 1 lines of equal specific gravity and index. On doing this we see that the reverse procedure is not possible, that is, knowing the specific gravity and the refractive indices we still cannot tell the composition, because the lines on the diagram are parallel or intersect at small angles. Simple qualitative chemical tests would serve to indicate the field in which some specimens would lie, but in most cases a quantitative analysis is necessary. In distinguishing rhodochrosites from minerals of the siderite-magnesite series the refractive index and specific gravity data may be useful. For a given nO the specific gravity of a magnesian siderite will be about 0.1 lower than for rhodochrosite. Having studied the relationships of composition, specific gravity, and refractive indices of a number of rhodochrosites from a wide variety of localities, it remains to be seen in what ways rhodochrosite from Butte is similar or different from other rhodochrosites. As suggested above, it was the erratic relationship between color banding and the optical data of Butte rhodochrosite that induced this study, and it is especially because of this that the following descriptions of occurrence, mineral association, texture and isomorphism of Butte rhodochrosite are considered of interest to this paper. RHODOCHROSITE AT BUTTE, MONTANA The Butte district has produced over 400,000 tons of high grade rhodochrosite ore since 1917. During the present emergency lower grade ores are being beneficiated by flotation. The rhodochrosite is calcined in large rotary kilns to produce an oxide which contains about 58 per cent manganese. Most of the ore has come from the Emma mine on the Black Chief lode near downtown Butte, but rhodochrosite is common in most of the veins of the southwestern and western portions of the district.13 The most abundant minerals with which the rhodochrosite is found are quartz, rhodonite, sphalerite, pyrite, and galena. Quartz is the dominant mineral in most veins of the peripheral zone. Rhodonite is abundant in the veins of the northern part of the district. Less common mineral associates of rhodochrosite are ankerite, calcite, barite, arsenopyrite, chalcopyrite, tetrahedrite, tenantite, chalcocite and bornite. Some of the ore shoots in the Emma mine consist almost exclusively of rhodochrosite. They seem to be localized by certain vein intersections and by flattening in the dip of the veins. Numerous smaller stringers of rhodochrosite are parallel to the main veins. Most of the walls of the rhodochrosite veins are tight and sharp. The wall rock of quartz monzonite is locally heavily sericitized, silicified and pyritized for short distances away from the veins. Rhodochrosite is one of the latest of the primary minerals in the peripheral zone. Fracturing and brecciation preceded and accompanied its deposition. Some minor faults tend to diminish and disappear in the carbonate. Quartz and sulphides are in general earlier than the rhodochrosite and occur in part as breccia fragments cemented by rhodochrosite. Later than rhodochrosite are a few small seams of dull milky quartz with sphalerite and pyrite. Locally there are vugs lined with euhedral rhombic crystals of rhodochrosite on some of which there is deposited a layer of tiny crystals of quartz and a few small pyrite and sphalerite crystals. In the northern part of the district rhodochrosite and rhodonite are essentially contemporaneous, but both thin sections and hand specimens commonly show rhodonite to be veined and partly replaced by rhodochrosite. Hence it is probable that rhodochrosite continued to form after rhodonite. Most of the rhodochrosite is banded, compact and crystalline, with grains ranging in size from 0.1 mm. to over 6 cm. The color ranges from deep rose to pale pinkish gray and cream. Many specimens are color-banded and some individual crystals are zoned. Some of the banding is textural, dependent on size of grain and the number and character of minute inclusions. It is evident that much of the rhodochrosite has been deposited in successive layers. About seventy determinations of refractive index of rhodochrosite grains of all colors, grain size and associations were made on specimens from all parts of the southwestern and western portion of the district. The lowest nO noted is 1.778 in the cream-colored band of a sharply-banded specimen from the 800 foot level of the Emma mine. The highest nO noted is 1.815 in dense pale pink rhodochrosite from the extreme western edge of the district. The great majority of the indices lie between 1.797 and 1.810, with the average at 1.8035. Geographic location within the district has no marked correlation with indices. In general the light or cream colored rhodochrosite bands are lower in index than the pink or rose carbonates. Some pale pink rhodochrosite bands or zones are lower in index than the deeper rose rhodochrosites but others are higher. There may be more variation in index within a color band than there is between adjacent color bands, though this is generally not true. Clearly there is considerable isomorphic variation in the Butte, rhodochrosite, Plate 1 shows the great range in isomorphous changes possible in rhodochrosites within the index range noted at Butte. Specimen N in Table 1 and Plate 1 is from the 1000-foot level of the Emma mine. It is a single, large, zoned crystal from a coarse-grained, aggregate and has a range in color from deep to medium pink. The determinations of specific gravity were made by J. G. Fairchild using the pycnometer method and by Waldemar T. Schaller using the Berman microbalance. The partial analysis by Fairchild is MnO 55.20, MgO 3.28, CaO 1.68, FeO 0.30, ZnO none, SO3 0.02, insoluble 0.05, CO2 39.31 (calculated); total 99.84 per cent. Two bulk analyses of Emma ore are available. Hewett and Pardee 14 give the average composition of about 250,000 tons of ore from the Emma mine and indicate the molecular composition of the rhodochrosite free from impurities to be MnCO3 91 per cent, MgCO3 7 per cent and CaCO3 2 per cent.The average percentage composition of 13,961 tons of rhodochrosite ore delivered to the Southern Manganese Corporation in 1918 and 1919 and the variation in monthly average analyses are given below:
If the FeO is assigned to pyrite rather than to FeCO3 following Hewett and Pardee, 15 the molecular composition of the rhodochrosite free from impurities is approximately as follows:
These analyses and the optical data indicate that Butte rhodochrosite is lower in iron and higher in magnesium than most rhodochrosites. The rhodochrosites of Philipsburg, Montana, specimens U, V, and W, have similar characteristics. OTHER MAGANESE MINERALS AT BUTTE In addition to the abundant rhodonite of the northern part of the district, minor quantities of mangan-ankerite, manganosiderite, 16 huebnerite,17 helvite18 and alabandite19 are found. A specimen of pale pink, massive, crystalline mangan-ankerite from the dump of the Bonanza mine was analyzed by J. G. Fairchild: CaO 30.52, MgO 15.92, MnO 4.28, FeO 3.31, ZnO none, SO3 none, insoluble none, and CO2 46.08 (calculated); total 100.11. The observed nO ranges between 1.690 and 1.698 and the calculated nO is 1.694 .The specific gravity and molecular proportions are given in Table 2 as specimen q.Greenish wolframite similar to that described as an alteration product of huebnerite by Winchell 20 was noted in a specimen of banded rhodochrosite from the Emma mine. The mineral occurs as extremely tiny, greenish prisms intimately intergrown with quartz. Spectrographic tests by K. J. Murata and chemical tests by Michael Fleischer showed the presence of Fe, Mn, and W. The identity of the wolframite was proved by x-ray powder pictures taken by W. E. Richmond.ACKNOWLEDGMENTS The writer is pleased to acknowledge the constructive criticism and helpful suggestions of Drs. Waldemar T. Schaller, D. F. Hewett, Charles F. Park, Jr., J. B. Mertie, Michael Fleisher, and Duncan McConnell. The Anaconda Copper Mining Company permitted the examination of the Butte carbonate ores. Persons responsible for the chemical and some of the optical data have been acknowledged throughout the text. The writer is indebted to Messrs. E. N. Goddard, W. S. Burbank, C. P. Ross, H. D. Miser, and D. F. Hewett of the U. S. Geological Survey for permission to include some of their analyses previously unpublished. NOTES * Submitted for publication with the approval of the director of the U. S. Geological Survey. 1 Ford, W. E., Studies in the calcite group: Trans. Conn. Acad. Arts and Sci., 22, 211-248 (1917).
2 Ford, W. E., op. cit., pp. 219-220. 3 Ford, W. E., op. cit., p. 218. 4 Krieger, Philip, Notes on an x-ray diffraction study of the series calcite-rhodochrosite: Am. Mineral., 15, 23-29 (1930). 5 Ford, W. E., op. cit., p. 221. 6 Ford, W. E., op. cit., p. 242. 7 Ford, W. E., op. cit., pp. 215-228. 8 Brentano, J., and Adamson, J., Precision measurements of x-ray reflexions from crystal powders: Philos. Mag., ser. 7, 7, 507-517 (1929). 9 Wyckoff, R. W. G., The crystal structure of some carbonates of the calcite group: Am.. Jour. Sci., 4th ser. 50, 317-320; 351-353 (1920). 10 Ewald, P. P., and Herman, C., Structurbericht 1913-1926, Zeits. Krist., 316-317 (1931). Also International Critical Tables, 1, 342 (1926). 11 Bragg, W. L., Atomic Structure of Minerals, Cornell Univ. Press, 114-116 (1937). 12 Wyckoff, R. W. G., The Structure of Crystals, 2nd ed., Am. Chem. Soc., Mon. Series 19, 275 (1931). 13 Hewett, D. F., and Pardee, J. T., Manganese in western hydrothermal deposits; Ore deposits of the western states (Lindgren Volume): Am. Inst. Min. Met. Engr., 672-675 (1933). Sales, R. H., Ore deposits at Butte, Montana: Trans. Am. Inst. Min. Met. Eng., 46, 3-109 (1914). Weed, W. H., The geology and ore deposits of the Butte district, Montana: U. S. Geol. Survey, Prof. Paper, 74 (1912). Hart, L. H., The Butte district, Montana; Copper resources of the world: XVI Int. Geol. Congress, 1, 287-305 (1935). Pardee, J. T., Deposits of manganese ore in Montana, Utah, Oregon, and Washington: U. S. Geol. Survey, Bull. 725c, 141-179 (1921). Pardee, J. T., Manganese at Butte, Montana: U. S. Geol. Survey, Bull 690e, 111-130 (1918). Perry, Eugene S., The Butte mining district, Montana: XVI Int. Geol. Congress, Guidebook 23 (1932). Bard, D. C., and Gidel, M. H., Mineral associations at Butte, Montana: Trans. Am. Inst. Min. Met. Eng., 46, 123-127 (1914). 14 Hewett, D. F., and Pardee, J. T., op. cit., p. 674. 15 Hewett, D. F., and Pardee, J. T., op. cit. p. 674. 16 Pardee, J. T., op. cit. (Bull. 690e), p. 119. 17 Winchell, A. N., Notes on tungsten minerals from Montana: Ec. Geol., 5, 158-165 (1910). 18 Hewett, D. F., Helvite from the Butte district, Montana: Am. Mineral., 22, 803-804 (1937). 19 Hewett, D. F., and Pardee, J. T., op. cit., p. 674. 20 Winchell, A. N., op. cit., p. 165.
|