1902 Encyclopedia > Justus Liebig
Justus Liebig
German chemist
(1803-73)
JUSTUS LIEBIG (1803-1873), was born at Darmstadt in 1803. His father carried on business as a drysalter and dealer in dye-stuffs, and made various experiments with a view to improved methods of preparing and purifying his wares. These led the son to take an interest in chemistry, and to seek for knowledge in the chemical books and periodicals in the grand-ducal library, which is rich in scientific works. At home he employed his time in repeating, as far as the means at his command admitted, the experiments he found described in books, and thus while still a boy attained a theoretical and practical knowledge of chemistry comparable with that of many full-grown professors of the science.

Justus Liebig
He determined to be a chemist, to devote his life to the pursuit of science. The only kind of chemist available for teaching purposes was the chemist and druggist, and accordingly Liebig, at the age of fifteen, entered the shop of an apothecary at Heppenheim near Darmstadt to study chemistry. He soon found out how great is the difference between practical pharmacy and scientific chemistry, and returned to Darmstadt, after ten months, to look for another and more likely way of attaining his object.
After some months spent in study at home he entered the university of Bonn, which he soon left for Erlangen. There he attended the lectures of Kastner on chemistry, and, besides the study of allied sciences, devoted some time to make up for the almost total neglect of school work caused by his early love of chemistry. He was much influenced by the metaphysical speculations of Schelling, and in after life referred to this influence as injurious to him as a scientific investigator.
In those days there were no laboratories accessible to ordinary students, and Liebig had to content himself with what the university could give him in the lecture-room and in the library. Both at Bonn and at Erlangen he formed a students' chemical and physical society for the discussion of new discoveries and speculations as these appeared in scientific books or periodicals. In 1822 he left Erlangen with the degree of Ph.D.
By means of the liberality of Louis I., grand-duke of Hesse-Darmstadt, Liebig was enabled to continue his chemical studies in Paris. There he made the acquaintance of Runge, Mitscherlich, and Gustav Rose. He attended the lectures of Gay-Lussac, Thenard, and Dulong, and, while carrying on the investigation into the composition and properties of the fulminates which he had already partly published, he attempted, as at Erlangen, to work up his neglected school studies. The results of his work on the fulminates were communicated to the Academy of Sciences, and attracted the favourable attention of Humboldt, who was at that time in Paris.
Humboldt introduced Liebig to Gay-Lussac, who admitted him into his private laboratory as a pupil. Here he had opportunities of learning all the mysteries of the art from one of the most skilful and ingenious of experimenters.
It was on the advice of Humboldt that Liebig determined to become a teacher of chemistry, but difficulties stood in his way. As a native of Hesse-Darmstadt, he ought, according to the academical rules of the time, to have studied and graduated at the university of Giessen, and Humboldt had to use his influence to induce the authorities to forgive his having attended the foreign university of Erlangen. After examination his Erlangen degree was recognized, and in 1824, in his twenty-first year, he was appointed extraordinary professor of chemistry in the university of Giessen.
Two years later he was promoted to the post of ordinary professor, which he held for twenty-five years, notwithstanding the most tempting offers from other universities. It was here, in the small town and small university of Giessen, that by far the most of Liebig's work was done.
He began by remedying the evil which as a student he had himself felt. He induced the Darmstadt Government to build a chemical laboratory in which any student of the university might obtain a thorough practical training. It is difficult for us, who live in a time when nearly every university and many schools possess well-arranged and often well-endowed laboratories, to understand how great a revolution was made in the practical teaching of physical science by the foundation of the Giessen laboratory. We can form some idea of it by reading Liebig's articles on the condition of chemistry in Austria and Prussia, in which he goes over in detail the means of teaching afforded in the various universities of those great countries. He tells us that in 1838 two young Prussians came to Giessen to study chemistry, unable to obtain entrance to a laboratory in their own country, but were ordered back again by the Prussian Government. Fortunately other Governments were less strict, or other students were less obedient, and crowds of young men anxious to study chemistry came to Giessen, and carried home the light there acquired. Partly by Liebig's urgent appeals to the interests and to the shame of the great German states, partly by the influence of his pupils, a great reform was effected, and German universities now vie with one another in offering opportunities of practical instruction in chemistry and the other physical sciences.
The amount and the importance of the laboratory work done by Liebig in Giessen were very great. Without considering here the work done by his students under his direction, of which no doubt a very large part was conceived by him, and in the execution of which he constantly contributed his assistance and advice, we shall look only at what appears under his own name. During the twenty-six years he spent at Giessen as ordinary professor, he contributed to scientific journals more than two hundred papers, about twenty of which were records of joint work, chiefly with Wohler. During the same time he published his works on organic analysis, organic chemistry, chemistry applied to physiology and agriculture, his Chemical Letters, and many smaller treatises. From 1832 he was joint editor of the Annalen der Pharmacie, from 1837 of the Handwörterbuch der reinen und angewandten Chemie, and from 1847 to 1856 of the Jahresbericht der Chemie. These statements give some idea of the amount of his work; of its importance and of its effect on the history of science we shall speak later.
In 1845 he was raised to the hereditary rank of baron under the title of Freiherr von Liebig. In 1852 he accepted the invitation of the Bavarian Government to the ordinary professorship of chemistry in the university of Munich. This office he held till his death in 1873.
In private life Liebig was hospitable, courteous, and kindly. Honoured by all the great scientific societies of the world, and regarded by almost every one as the great authority in chemistry, he assumed no airs of superiority, and lived the simple and quiet life of a German professor. Liebig's influence on the history of chemistry may be considered under five heads :—(1) the effect of the opening of the Giessen laboratory, and of Liebig's constant efforts to induce other universities to follow this example; (2) the improvements introduced by him in methods of investigation and in apparatus; (3) the discovery of new facts; (4) the development of theory; and (5) the application of chemistry to physiology, agriculture, and the arts.
We have already spoken of the first. Under the second head by far the most important change introduced by Liebig was his method of organic analysis. Organic substances were analysed, and analysed with accuracy, before 1830, but such analyses could then be carried out only by highly skilled chemists, and involved great labour and the use of cumbrous apparatus. Liebig's method of organic analysis, which was published in all its details in 1831, and which (with important but secondary improvements) is that still used, made it easy for any advanced student to make a fairly accurate and very useful analysis of an organic substance. Analysis is to the chemist what astronomical methods for determining longitudes and latitudes are to the geographical explorer. Without it many interesting and useful discoveries may be made, but it is only when complete and accurate analyses are made of all the new substances produced in the course of a research that the research becomes fully available to other explorers. If Liebig had contributed nothing to organic chemistry but his method of analysis, he would still have been in a perfectly true sense the founder of modern organic chemistry. Many other improvements of apparatus are due to him; we need only mention the simple form of condenser called by his name, and constantly used by every chemist, and the easy and accurate method for determining the quantity of urea in a solution, which was the first step towards introducing precise chemical methods into practical medicine. This is also the proper place to refer to his analyses of the natural alkaloids, and his discovery of the method of determining their equivalents by the analysis of their chloroplatinates. In the third place we have to consider the new facts discovered by Liebig. The very great addition to our knowledge of organic chemistry made by Liebig naturally throws into the shade his contributions to inorganic chemistry, but we ought to remember his numerous analyses of mineral waters and his contributions to the difficult question of the accurate separation of cobalt and nickel. It is, however, in organic chemistry that Liebig's great discoveries were made. These discoveries are so intimately connected with his chemical theories that we may most conveniently consider them along with the fourth head, his contributions to the development of chemical theory.
The notion of compound radicals is to be found in chemistry as far back at least as the time of Lavoisier. Lavoisier says, " Some experiments of my own and some made by M. Hassenfratz have convinced me that in general nearly all the vegetable acids, such as tartaric, oxalic, citric, malic, acetic, pyrotartaric, pyromucic acids, have for their radical hydrogen and carbon, but united so as to form a single base, that all these acids differ from one another by the difference in proportion of these two substances and the degrees of oxidation." Berzelius adopted this view and expressed it thus,— ''We find the difference between organic and inorganic bodies to be that, while in inorganic nature all the oxidized substances have a simple radical, all the organic substances have compound radicals. Just as ammonia is an alkali with a compound radical, but possessing nevertheless the greatest analogies with the mineral alkalies which have simple radicals, so we find the greatest analogy between the mineral and the organic acids, so that the relations of potash and soda to acetic, oxalic, citric acids are the same as their relations to sulphuric, nitric, phosphoric acids." These views were published by Berzelius in 1817. In 1815 Gay-Lussac had discovered cyanogen, and shown that this compound of carbon and nitrogen is the radical of prussic acid and its salts, in the same sense as chlorine is the radical of hydrochloric acid and the chlorides. Ampere had indicated a theory of the constitution of the ammonia salts, which Berzelius worked out in detail, according to which those salts contain a compound radical, ammonium, playing in them the part of potassium in the potash salts. Finally, Davy suggested in 1815 that the hydrated acids which correspond in function to hydrochloric acid should be regarded as the true acids, and proposed to represent them as compounds of hydrogen with a radical.
Such were the knowledge and the theoretical position of chemists on this question when Liebig became professor; the dates to which we have referred correspond to the time when as a schoolboy he was devouring the contents of chemical journals at Darmstadt, and no doubt he then became acquainted at first hand with the discoveries and speculations of Ampere, Davy, Gay-Lussac, and Berzelius.
We have seen that his first investigation referred to the fulminates. He continued his researches upon the compounds of evanogen, and the substances connected with them formed frequent subjects of his researches during his whole life. In this region of organic chemistry he made many important discoveries, of which the limits of this article do not allow a detailed account; we can only mention melone, mellam, ammeline, ammelide, and melanine, as substances discovered and investigated by him. In the course of these investigations he discovered the precise nature of the chemical changes which occur in the manufacture of bromide of potash. In 1831 he examined the action of chlorine upon alcohol, among other substances discovered chloral, and commenced the series of investigations into the derivatives of alcohol and ether to which we shall refer immediately. In 1832 Wohler and Liebig published the results of their joint research on the oil of bitter almonds and its derivatives. This research may be said to stand at the head of modern organic chemistry. For elegance of method and for clearness of insight it is unrivalled, and will always remain a model of what such an investigation should be. They showed in the clearest manner that the compound C7H50 (here and elsewhere in this article we use the symbols now in common use, instead of those employed by the authors), to which they gave the name of benzoyl, is the constant part, or radical, of a great series of compounds. The importance of this investigation was generally recognized. Berzelius hailed it as the advent of real daylight on the subject of organic chemistry, and even suggested the names proin or orthrin (from proi [Gk.] and orthos [Gk.]) for the newly discovered radical.
We now come to the long controversy as to the constitution of alcohol and ether, which engaged so much of Liebig's time and energy. Gay-Lussac had shown in 1815 that alcohol and ether might, as far as their composition is concerned, be represented as compounds of defiant gas and water, and further that, if we represent the quantities by volume of gas or vapour, ether contains one volume of defiant gas and half a volume of water vapour, while alcohol contains equal volumes of the two. In 1828 Dumas and Boullay published an elaborate memoir on the preparations and properties of the ethers, in which they further developed the ideas suggested by Gay-Lussac. They regarded defiant gas as the radical of all the etherial compounds, as ammonia is of the ammoniacal salts, and formulated them thus:—
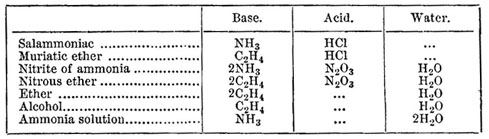
We have given only a sample of their tables, leaving out among others some substances in reference to the composition of which they had fallen into error, error which Liebig detected and used as an argument, valid enough then no doubt, but of little interest now.
In 1833 Liebig proposed a quite different theory one which stands to Dumas and Boullay's in the same relation which the ammonium theory holds to the ammonia theory of the constitution of the ammoniacal salts. Just as Ampere and Berzelius regarded sal-ammoniac, not as NH3,HCl, but as NH4,Cl, so Liebig proposed for muriatic ether the formula C2H5,Cl instead of C2H4,HCl. The really cogent argument which he brought forward, the argument which we can now best appreciate, is that, while alcohol contains combined water, ether does not. According to Dumas and Boullay alcohol and ether are both hydrates, but in Liebig's view ether is (C2H5)2O and alcohol (C2H5)2O,H2O. If we wish to understand this argument we must recollect that to the chemists of that time oxidized hydrogen was water, and Liebig's arguments are as sound now as they were then, for the most recent views represent alcohol as C2H5—O—H, and this contains oxidized hydrogen, or, as we may say, half a molecule of water, and Liebig's tests do not distinguish preformed water, but hydrogen and oxygen combined as they are in water. Much debate and investigation followed, in the course of which the relations of all the substances derived from alcohol were thoroughly and practically studied in a fuller and more careful manner than would have been possible had there not been a theoretical point to defend and to attack. The enormous number of facts discovered by Liebig put him in a very favourable position as the advocate of his theory. Chemists could not do without a knowledge of these facts, and they could only get this knowledge through Liebig's papers, in which the facts were expressed in the language of his theory.
In 1835 Regnault began a series of most important researches into the compounds derived from defiant gas. He showed that many of these substances might be classified and their relations explained by the assumption of a radical C2H3, to which he gave the name of aldehydene. Liebig saw that according to his view of the meaning of the word radical, as "the unchanging constituent in a series of compounds," the same radical may be assumed in alcohol and in acetic acid, and in 1839, in a note published in the Annalen, he puts the matter in a very clear light. He says, '' Ether and ammonia have in their compounds a certain resemblance which was first indicated by Dumas and Boullay, and the view of the constitution of the ammonia salts which is generally held in France was the reason why ether was considered the first hydrate of defiant gas, alcohol as the second hydrate, &c. ; in Germany and other countries the water necessary for the constitution of the salts of ammonia with oxygen acids was considered as an integral part of the base.; it was assumed that this water forms with the ammonia oxide of ammonium (NH4)2O, and this view in a certain sense smoothed the way for another, according to which the existence of organic oxides, capable of neutralizing acids, appeared very probable, as a necessary complement to the organic acids which chemists had long been inclined to regard as oxides of organic radicals. Ether was in these countries regarded as an organic oxide, and this difference of view excited a ten years' strife, as an immediate result of which we may regard the discovery of a great number of compounds which enriched science with innumerable important observations. No region of organic chemistry has been so thoroughly and so completely studied as the compounds connected with ether ; and now, when the existence of organic oxides is no longer denied, the support of the opposite opinion has come to an end, although it can not be said that the q uestion itself has been experimentally decided. If we compare in the light of our present knowledge the ammonia compounds with the ether compounds, we at once see that the opposing views were fundamentally the same in the two cases. The disputes took place because we were not at one as to the interpretation of the phenomena. The ether and ammonia compounds assume in fact the same form of amidogen is regarded as the unchangeable radical of the ammonia compounds, and acetyl [Regnault's aldehydene] as the starting point of the ether compounds. The two sets of compounds differ only in so far as we must ascribe to acetyl the power of forming acids, a power which amidogen does not possess."
He then gives a table containing in two columns the ammonia and the ether compounds, in which C2H3 corresponds to NH2, C2H4 to NH3, and C2H5 to NH4, and adds, "These formula; require no explanation ; they have been developed in order to show the extraordinary resemblance of the ammonia and the ether compounds, and to show why it was that many chemists regarded olefiant gas as the first member of the series of ether compounds. Both of the formerly antagonistic theories have, as may be easily seen, from this point of view the same foundation, and all further questions as to the truth of the one or other view is thus of course set at rest."
It was during the course of the controversy which then closed that Liebig had discovered aldehyde, chloral, and, simultaneously with Soubeiran, chloroform, besides numerous other substances of less general interest, and developed the theory now generally received of the formation of ether by the action of sulphuric acid on alcohol.
In the very short sketch given above of the discussion as to the constitution of ether, we mentioned that Liebig's ethyl theory was to some extent borrowed from Berzelius. Berzelius had suggested that ether was the oxide of a radical (Liebig's ethyl), but he was at first inclined to regard alcohol, not as the hydrate oxide of the same radical, but rather as the oxide of another one, which with our symbols would be written C2H6. But there was a deeper difference than this between the radical theories of Berzelius and Liebig. This essential difference first clearly showed itself in the notes which Liebig added to two letters from Berzelius to Wohler, published in the Annalen in 1839. In these letters Berzelius gives his views of the constitution of oxychlorides, with whieli he classes such bodies as trichloracetic acid. All those bodies he represents as compounds of oxides and chlorides, in harmony with the dualistic system.
Thus, instead of SO2Cl2, C2HCl3O2, &c, he writes SCl6+2SO3, C2Cl6+C2O3, &c. (taking the anhydrous acid). In his second letter on Malaguti's chlorinated ethers he naturally arrives at formulae of extreme complexity. In his notes Liebig states that he does not agree with Berzelius, and that the analogy first pointed out by Berzelius between inorganic and organic compounds and his theory of organic radicals had been a guiding star in a labyrinth in which previously no one could find the way. " But, while there are points of resemblance, there are very many points of difference; we should follow a theory as long as it gives us light and explains facts; up to a certain point the principles of inorganic chemistry help us in organic chemistry, beyond it they leave us, and produce instead of removing complications; beyond this point we require new principles."
These new principles were supplied by Liebig's radical theory. As Liebig showed, abstract discussions as to the truth of a theory are out of place in an experimental science; the question is not as to their essential truth but as to their practical fruitfulness. Do they help us to understand old and to discover new facts? If they do, the morrow may take thought for its own theories. These are not Liebig's words, but they seem to express his ideas.
Liebig early expressed his approval of Davy's views as to the constitution of acids, but he rarely used the language or the notation corresponding to that view. This divergence between his theory and his habitual language is interesting as showing that he held that the same truth may be expressed in more than one way, and that where no immediate point is to be gained it is well to employ the language best understood by those whom we address. In this, as in his preference for what was called the equivalent system of notation over that of Berzelius, he showed his sound practical judgment and common sense. We now see that the notation of Berzelius was nearer the truth, but its advantages could not be felt until chemistry had advanced further, and its retention would have led to complications of formuhe and obscuring of relations. The resemblance indeed of our present notation to that of Berzelius is to a great extent accidental, and the advance was hastened rather than hindered by what now looks like a retrograde step.
There is one other point which we have to mention under the present head. Liebig at once saw the importance of Graham's researches on the phosphates. He applied Graham's idea of poly-basicity to organic acids, and satisfactorily proved, notwithstanding the opposition of Berzelius, that tartaric acid is dibasic and citric acid tribasic.
We have hitherto said nothing as to the relation of Liebig's theories to those at present held by chemists. On this subject a word may suffice. The great revolt against the radical theory led by Laurent and Gerhardt produced a long and acrimonious controversy. In that controversy Liebig took his part, and many hard and some unfair things were said by him. The controversy itself was of course the means of producing a vast amount of thorough research, and was thus, like all such contests, of direct use; it also led to the revision of all theoretical opinions from a totally new point of view. From this ordeal the radical theory has emerged, not very different in appearance. But it has undergone a profound change. Its foundations have been immensely strengthened, it has been to a great extent explained. Some chemists seem to think that this makes it an entirely new theory. We cannot share this view. Our reasons for believing in ethyl and benzoyl differ from the reasons adduced by Wohler and Liebig only in this that we have arguments which they had not; their arguments remain. We now know something of the reason why such radicals exist; we can, to a certain extent, deduce their properties from those of the elements which they contain, but explanation is something different from refutation ; the theory has grown, but still remains the same theory.
Liebig all his life showed a special predilection for the study of the immediate products of animal life. He investigated with untiring zeal the substances contained in urine and in the juice of flesh. In these researches he discovered many new substances, and cleared up doubts and difficulties as to their relations to one another and to other bodies. Late in life he expressed the most lively interest in Volhardt's synthesis of sarcosin and creatine, substances with the preparation of which he had long before been engaged. In this connexion it is right that we should mention his elaborate investigation on uric acid and its derivatives. This line of study led him to interest himself in the chemistry of food, and the importance of his work in this direction can scarcely be overestimated. We do not refer alone to his methods of preparing the extract of meat and the food for infants, which have perhaps spread his fame more widely than his strictly scientific work could have done; we refer rather to the influence which his analyses and calculations have had on medical opinion and practice. And this leads us to our fifth head, Liebig's influence on the application of chemistry to physiology, agriculture, and the arts.
Before Liebig undertook his investigations into the chemistry of vegetation, the views (they can scarcely be called theories) held as to the manner in which plants are nourished were vague in the extreme. The only point satisfactorily made out was that under the influence of light the green parts of plants are capable of decomposing carbonic acid, giving off oxygen and retaining the carbon. Saussure, to whose careful experiments the establishment of this fact, first noticed by Priestley, is mainly due, believed that the nitrogen of the plant came from soluble organic substances absorbed by the roots, and expressly says that the main use of ammonia in manure is as a solvent of humus, which he supposed to be one source of the carbon in plants ; and, although the ashes of the various plants had been analysed, the importance of the mineral constituents of vegetables was not at all recognized. Liebig undertook the investigation of this question in 1840. He showed that the plant derives its nourishment partly from the air, partly from the soil ; the carbonic acid and water, the ammonia and nitric acid, which he showed to be the sources of the plant's nitrogen, come from the atmosphere ; while the potash, soda, lime, iron, magnesia, sulphuric acid, phosphoric acid, and silica come from the soil. No exhaustion can take place of the former, but the soil contains only a limited amount of the latter in a soluble state, and when this is used up the soil becomes barren. Not only so, but the absence of any one of the necessary substances makes the soil barren. He showed how manure acts by restoring these deficient ingredients, and how, when the land is left fallow, atmospheric influences decompose the insoluble minerals and supply the soil with what has been removed. He further showed that plants use and therefore remove from the soil the articles of plant food in various proportions, and thus explained the advantage of a rotation of crops. The artificial manures which he introduced contained the essential mineral substances, and a small quantity of ammoniacal salts, because he held that while the air supplies ammonia it does not always supply it fast enough, particularly to the less leafy plants. He bought a field near Giessen for his experiments, and treated it with the artificial manure, but the result was disappointing. The manure was not inactive, but not nearly so active as it should have been. It was many years before he detected the cause. To prevent the rain washing away the alkalies in the manure, he had taken great pains to render them insoluble. Way's experiments on the absorption of manure by soils (1850) occurred to him as suggesting an explanation, and in 1857 he made a number of experiments on the retention of various substances by earth. In these he confirmed and extended Way's observations, and thus saw that his effort to make his manure better had made it worse. As he says, "I had sinned against the wisdom of the Creator, and received my righteous punishment. I wished to improve His work, and in my blindness believed that, in the marvellous chain of laws binding life on the earth's surface and keeping it always new, a link had been forgotten which I, weak powerless worm, must supply."
Now, just as he showed that plants require certain—often small— quantities of particular substances, else they will not grow at all, however great maybe the quantities of other kinds of food supplied, so he showed that animals also require, not only a proper quantity of food, but also the right proportion of the different kinds of food, mineral as welt as organic. In the classification of the kinds of organic food into heat-producing and blood-forming, it was necessary to examine whether the carbohydrates, starch, sugar, &c, should be placed alongside of fat. He was thus led to inquire into the power of the animal body to produce fat from starch or sugar, and came to the conclusion, contrary to the opinion of Dumas and Boussingault, that this transformation does take place.
Liebig's investigations into the relations of organic chemistry to physiology led him to the conviction that the only source of animal heat is the heat produced by the oxidation of the tissues, and, strange as it may appear, he had to defend this view against what lie truly enough, though perhaps somewhat impolitely, called the absurd nonsense of his medical opponents. He also succeeded in demolishing, it is to be hoped finally, the ridiculous belief in the possibility of the spontaneous combustion of the human body.
Liebig's theory of FERMENTATION (q.v.) aimed at explaining the phenomena on purely chemical principles. He ridiculed the physiological theories, and looked upon the growth of fungi rather as incident of the fermentation, adducing the fermentative changes of amygdaline and similar substances as cases of fermentation without life.
We have still to notice one of Liebig's chemical discoveries, of secondary interest chemically, but of great practical importance. This is his discovery of a method for depositing a uniform film of metallic silver on smooth clean surfaces. This method may render it possible to use reflectors for astronomical telescopes of a size unattainable with the old speculum metal.
The most important of Liebig's works separately published are as follows:— Anleitung zur Analyse organischen Körper, 1837, 2nd ed.. 1853; Die Chemie in ihrer Anwendung auf Agricultur und Physiologie, 1810, 9th ed., 1875-76; Die Thier-Chemie oder die organische Chemie in ihrer Anwendung auf Physiologie und Pathologie, 1812, 3rd ed, 1847 ; Handbuch der Chemie mit Rücksicht auf Pharmacie, 1843; vol. i. of Gelger's Handbuch der Pharmacie, new ed.; Chemische Briefe. 1844, 6th ed., 1878 ; Chemische Untersuchungen über das Fleisch und seine Zubereitung als Nahrungsmittel, 1847; Grundsätze der Agricultur-Chernie, 1850; Zur Theorie und Praxis in der Landicirthschaft, 1856; Naturwissenschaftliche Briefe über die moderne Landwirthschaft, 1859; Reden und Abhandlungen, 1874, a posthumous collection of some of his addresses and shorter publications. Liebig's scientific papers were chiefly published in Foggendorff's Annalen till 1839, and in Liebig's Annalen from 1832 onwards. His criticism of Bacon appeared in the Augsburger allgemeine Zeitung in 1863 and 1864, and also as a separate publication. (A. C. B.)
The above article was written by Alexander Crum Brown, M.A., M.D., D.Sc., LL.D.., F.R.S.; Professor of Chemistry, Edinburgh University, from 1869; President of Chemical Society of London, 1892-93; author of The Development of the Idea of Chemical Composition.
|

